
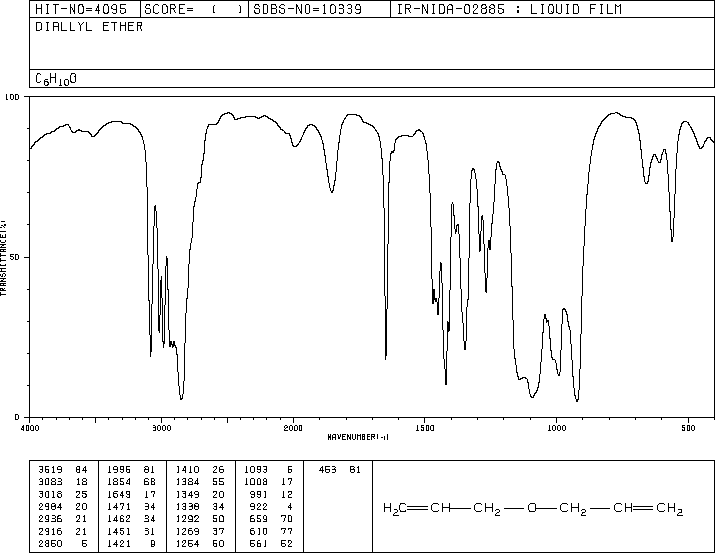
If you look at an IR spectrum of dibutyl ether, you will see: there are the usual sp 3 C-H stretching and CH 2 bending modes at 29 cm-1. The (B) protons in turn are coupled to a set of two and three equivalent protons and you would therefore formally expect a quartet of triplets. An oxygen atom could be found in between two carbons, as in dibutyl ether. So, each of these signals appears as a triplet. When analyzing an IR spectrum, it is helpful to overlay the diagram below onto the spectrum with our mind to help recognize functional groups. Protons at (A) and (C) are each coupled to two equivalent (B) protons. Notice the protons closer to the electron withdrawing oxygen atom are further downfield indicating some deshielding. The 1H NMR spectrum of dipropyl ether shows three signals with the triplet at 3.37 ppm assigned to the -CH 2- beside the ether and the other two signals upfield (1.59 and 0.93 ppm). Hydrogens on carbons in and epoxide show up at 2.5 to 3.5 ppm. Acetaldehyde, ethanol, and dimethyl ether. Similar peaks in epoxides are shifted to a slightly higher field than other ethers. Infrared spectra of complex organic molecules in astronomically relevant ice matrices. The obvious way to know a molecule is an ether is to see a C-O peak, but no CO or O-H, since the absence of a CO or O-H stretch confirms it is not an ester, acid, or alcohol.Hydrogens on carbon adjacent to the ether show up in the region of 3.4-4.5 ppm.To find out more information about our offerings, please contact us at Names: BFM, bromofluoromethylene, CFC 31B1, R 31B1ġH NMR Spectra (CDCl3): 6.1 (d, J(F-H) = 48 Hz, 2H) ppmġ3C NMR Spectra (CDCl3): 77.4 (d, J(C-F) = 247 Hz) ppmġ9F NMR Spectra (CDCl3): -163.5 (t, J = 48.9 Hz) ppm Because hexane has only C-H and C-C bonds (and no functional groups), this spectrum can help orient you to the important regions in an IR spectrum. The IR spectrum of hexane (C 6 H 14) is shown in the next figure. Using proprietary technology, Valliscor manufactures kilogram to commercial scale quantities of BFM for the pharmaceutical industry at its Corvallis, Oregon production facility. This table lists the locations and intensities of absorptions produced by typical functional groups. Methoxy, methyl ether O-CH 3, C-H stretch 28502815 Methylamino, N-CH 3, C-H stretch 28202780 Figure 1. Valliscor Capabilities for Bromofluoromethaneīromofluoromethane (BFM) is an important building block primarily used in the commercial manufacture of the APIs fulticasone propionate and fuliticasone furoate. The schematic IR spectrum is available in Figure 1, and the specific frequency of each.


 0 kommentar(er)
0 kommentar(er)
